[ad_1]
January was cold and blue again, but at least this year it brought snow to rainy Manchester. One day, a snowman appeared outside the library and slush formed on the road, which made us wonder about snow, especially the impressive formation of snowflakes when it falls. If you too have ever been interested in the answer to how such intricate patterns arise naturally, here is a how-to manual for building delicate ice crystals.
From dust to snowflakes
The formation of a snowflake involves several different steps determined by a combination of mathematical probabilities, atmospheric changes, chemical bond angles, and, as always, the right physics.
First of all, a snowflake needs a starting point. Tiny dust and pollen particles in the atmosphere provide it. In the glacier winter sky, water molecules freeze around these particles, forming tiny ice crystals that form the basis of snowflakes.
The next step is the placement of the hexagonal core. Water molecules freeze together to form ice sheets, with hydrogen bonds forming between each molecule. The bond angle between two hydrogen atoms in water is 104.5 degrees, so the molecule forms a hexagonal shape. And when these hexagons combine, they begin to form larger hexagons.
This process continues. The hexagons bond evenly because a new water molecule has an equal chance of colliding with any of the six symmetric hexagon sides. In this way, the iconic snowflake-shaped nucleus is formed.
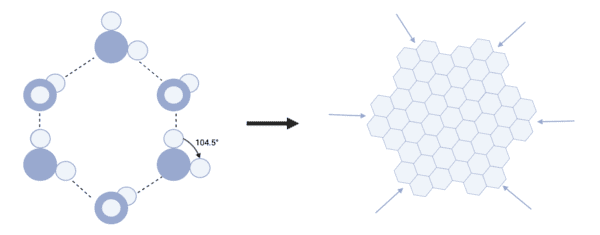
The final consideration is how the rest of the snowflake, with its intricate pattern of connected arms, will be created. The bifurcation occurs because his six vertices of the hexagonal core protrude beyond the sides and are therefore more likely to interact with surrounding water molecules and form hydrogen bonds. This increases the growth at these points and forms six symmetrical arms. As water molecules continue to collide, smaller branches grow from these main branches, forming complex, symmetrical patterns.
unique beauty
Although these steps describe the basic template of a snowflake, in reality it is well known that the exact shape of each snowflake is unique and determined by the surrounding temperature and conditions at the time of formation. It is being The shape of a growing snowflake is so sensitive and capricious that even small changes in temperature can change its course mid-formation.
Nevertheless, the states of each of the six sides of a snowflake are always similar enough to make the snowflake symmetrical. No two snowflakes share the same path to the ground. This means that on each long journey through the air, conditions are slightly different, resulting in a unique arrangement of water molecules. Given the number of possible branching points within a growing flake and the probability that identical environmental conditions exist, it is very likely that the snow flake pattern will never repeat.
So the next time you spot a snowflake on your coat, take a moment to appreciate its unique beauty. If your flake looks particularly strange, know that it may have experienced turbulence during its fall.
[ad_2]
Source link


