[ad_1]
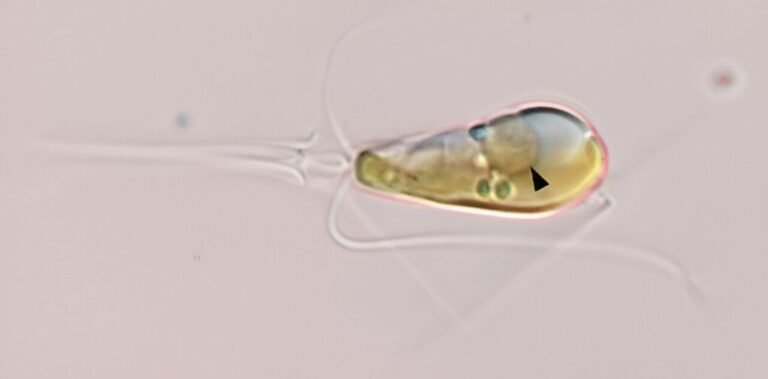
Light microscopy image shows the marine haptophyte alga Braarudosphaera bigellowii with a black arrow pointing to the nitroplast organelle.Credit: Tyler Cole
× close
Light microscopy image shows the marine haptophyte alga Braarudosphaera bigellowii with a black arrow pointing to the nitroplast organelle.Credit: Tyler Cole
Modern biology textbooks claim that only bacteria can take nitrogen from the atmosphere and convert it into a form that can be used by life. Plants such as legumes fix nitrogen by harboring symbiotic bacteria in their root nodules. But recent discoveries have upended that rule.
In two recent papers, an international team of scientists describes the first known nitrogen-fixing organelles in eukaryotic cells. This organelle is his fourth example in the history of primary endosymbiosis, a process in which prokaryotic cells are engulfed by eukaryotic cells and evolve beyond symbiosis into organelles.
“It’s very rare for organelles to develop from these types of materials,” says Tyler, a postdoctoral fellow at the University of California, Santa Cruz, and first author of one of the two recent papers.・Mr. Cole says. “The first time it is thought to have happened, it gave rise to all complex life. Everything more complex than a bacterial cell exists thanks to its existence,” he said, referring to the origin of mitochondria. . “About a billion years ago, the same thing happened with chloroplasts, which gave us plants,” Cole said.
The third known example involves microorganisms that resemble chloroplasts. The latest discovery is the first example of a nitrogen-fixing organelle, which researchers call a nitroplast.
A decades-old mystery
The discovery of this organelle required a bit of luck and decades of research. In 1998, Jonathan Zehr, Distinguished Professor of Marine Science at the University of California, Santa Cruz, discovered a short DNA sequence in Pacific ocean water that appeared to come from an unknown nitrogen-fixing cyanobacterium. Zehr and his colleagues spent years studying a mysterious creature they called UCYN-A.
Around the same time, Kyoko Hagino, a paleontologist at Kochi University in Japan, was enthusiastically working on cultivating seaweed. It turned out to be the host organism for UCYN-A. It took more than 300 sampling expeditions and more than 10 years of her time, but Hagino finally succeeded in culturing the algae and, together with other researchers in the lab, developed her UCYN-A and its You can now begin researching marine algae hosts.
For many years, scientists thought that UCYN-A was an endosymbiont closely related to algae. However, two recent papers suggest that UCYN-A has coevolved with the host through past symbiosis and now fits organelle criteria.
Origin of organelles
In a paper published in cell In March 2024, Zea and colleagues at the Massachusetts Institute of Technology, the Barcelona Institute of Science, and the University of Rhode Island showed that the size ratios of UCYN-A and its algal host are similar among different species of marine haptoalgae. Ta. Braardosfaela Bigelowi.
The researchers used their model to demonstrate that the growth of host cells and UCYN-A is controlled by an exchange of nutrients. Their metabolism is linked. This synchronized growth rate led the researchers to call UCYN-A an “organelle-like thing.”
“That’s exactly what’s happening in organelles,” Zea says. “If you look at mitochondria and chloroplasts, it’s the same thing. They change to suit the cell.”
Soft X-ray tomography images show cell division of B. bigellowii with cyan-colored nitroplasts (UCYN-A).Credit: Valentina Loconte
× close
Soft X-ray tomography images show cell division of B. bigellowii with cyan-colored nitroplasts (UCYN-A).Credit: Valentina Loconte
But scientists didn’t confidently call UCYN-A a cell organelle until they saw other lines of evidence.Magazine cover article sciencePublished today, Zea, Cole, Kendra Turk Kubo, and Wing Kwan Esther Mak of the University of California, Santa Cruz, and the University of California, San Francisco, Lawrence Berkeley National Laboratory, and National Taiwan Ocean University. , co-researchers at Kochi University in Japan, show that UCYN-A imports proteins from host cells.
“This is one of the characteristics of when something moves from an endosymbiont to an organelle,” Zea says. “They start throwing away bits of DNA, the genome becomes smaller and smaller, and they start relying on the mother cell to transport those gene products, or the proteins themselves, into the cell.”
Cole worked on proteomics for his research. He compared the proteins found within his isolated UCYN-A to those found throughout the algal host cell. He discovered that host cells make proteins and label them with specific amino acid sequences that instruct the cells to send the proteins to the nitroplast. The nitroplast then imports and uses the protein. The calls identified the functions of several proteins that filled gaps in specific pathways within UCYN-A.
“It’s like a magical jigsaw puzzle that actually comes together and works,” Zehr said.
In the same paper, UCSF researchers showed that UCYN-A replicates synchronously with algal cells and is heritable like other organelles.
change perspective
These independent lines of evidence leave little doubt that UCYN-A goes beyond a symbiotic role. And while mitochondria and chloroplasts evolved billions of years ago, nitroplasts appear to have evolved about 100 million years ago, providing scientists with a new, more recent perspective on organogenesis. .
This organelle also provides insight into marine ecosystems. All living organisms require nitrogen in a bioavailable form, and UCYN-A is of global importance for its ability to fix nitrogen from the atmosphere. Researchers have found this substance everywhere from the tropics to the Arctic Ocean, where it fixes large amounts of nitrogen.
“He’s more than just a player,” Zehr said.
This discovery also has the potential to change agriculture. The ability to synthesize ammonia fertilizer from atmospheric nitrogen enabled agriculture and the world’s population to take off in the early 20th century. This process, known as the Haber-Bosch process, enables about 50% of the world’s food production. It also generates huge amounts of carbon dioxide. Approximately 1.4% of global emissions result from this process. Researchers have been trying to figure out how to incorporate natural nitrogen fixation into agriculture for decades.
“This system represents a new perspective on nitrogen fixation and may provide clues as to how such organelles can be incorporated into crops,” Cole said.
However, many questions regarding UCYN-A and its algal host remain unanswered. The researchers plan to dig deeper into how UCYN-A and algae work and study different strains.
Kendra Turk Kubo, an assistant professor at the University of California, Santa Cruz, will continue her research in her new lab. Zehr expects that scientists will discover other organisms with similar evolutionary stories to UCYN-A, but the discovery is the first of its kind, and the discovery is not textbook-worthy. It’s a discovery.
For more information:
Tyler H. Coale et al, Nitrogen-fixing organelles of marine algae, science (2024). DOI: 10.1126/science.adk1075
Francisco M. Cornejo-Castillo et al. Metabolic tradeoffs constrain cell size ratios in nitrogen-fixing symbioses, cell (2024). DOI: 10.1016/j.cell.2024.02.016
Magazine information:
science
,cell
[ad_2]
Source link