[ad_1]
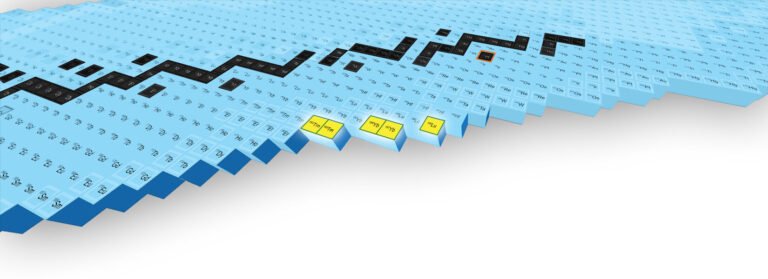
Report on the production of new isotopes in magazines physical review letter, scientists are one step closer to being able to more directly investigate the natural processes that create new elements in stars. New isotopes can also help improve and inform our understanding of fundamental nuclear physics. Credit: FRIB/MSU
× close
Report on the production of new isotopes in magazines physical review letter, scientists are one step closer to being able to more directly investigate the natural processes that create new elements in stars. New isotopes can also help improve and inform our understanding of fundamental nuclear physics. Credit: FRIB/MSU
An international research team working at Michigan State University’s Rare Isotope Beam Facility (FRIB) has brought a star closer to Earth by creating five new isotopes.
The isotopes known as thulium-182, thulium-183, ytterbium-186, ytterbium-187, and lutetium-190 are reported in the journal. Physical review letter.
These are the first batch of new isotopes produced at FRIB, a U.S. Department of Energy Office of Science (DOE-SC) user facility that supports the mission of the DOE-SC Office of Nuclear Physics. The new isotopes indicate that FRIB is moving closer to creating nuclear samples that currently exist only when ultra-dense objects known as neutron stars collide.
“That’s the exciting part,” said Alexandra Gade, professor of physics in FRIB and MSU’s Department of Physics and Astronomy and FRIB scientific director. “We are confident that we can get even closer to the atomic nucleus, which is important for astrophysics.”
Gade is also a co-spokesperson for the project, which is led by FRIB senior research physicist Oleg Tarasov.
In addition to cohorts based at FRIB and MSU, the research team also included collaborators from Korea’s National Institute for Basic Science and Japan’s RIKEN (abbreviation for RIKEN).
“This is probably the first time these isotopes have been present on the Earth’s surface,” said Bradley Sherrill, University Distinguished Professor in MSU’s College of Natural Sciences and director of FRIB’s Division of Advanced Rare Isotope Separations. said.
As for what “advanced” means in this context, Sherrill said researchers are using FRIB’s state-of-the-art equipment to produce two particles of a new isotope, in order to confirm their existence and identity. He said he only needed three.
Because researchers know how to create these new isotopes, they can now create them in large quantities to perform experiments that were previously impossible. The researchers are also keen to follow in their footsteps to create more new isotopes that are more similar to those found in stars.
“I like the analogy of a journey. We were looking forward to going somewhere we’ve never been before, and this is the first step,” Cheryl said. “We’re leaving the house and starting exploring.”
Almost like a star
Our sun is the atomic factory of the universe. This is powerful enough to take out the nucleus, or nucleus, of two hydrogen atoms and fuse them into one helium nucleus.
Hydrogen and helium are the first and lightest items on the periodic table of elements. Getting to the heavier elements on the table requires an even harsher environment than what is found under the sun.
Scientists hypothesize that when two neutron stars merge, a gold-like element (about 200 times more massive than hydrogen) will be produced.
A neutron star is the leftover core of an exploded star, originally much larger than the Sun, but not large enough to eventually become a black hole. Neutron stars are not black holes, but they pack a huge amount of mass into a very modest size.
“They are the size of Lansing and roughly the mass of the sun combined,” Sherrill said. “People think, although we’re not sure, that all the gold on Earth was created in neutron star collisions.”
By producing isotopes present at neutron star collision sites, scientists will be able to better investigate and understand the processes involved in producing these heavy elements.
Although the five new isotopes are not part of that environment, they are the closest scientists have come to reaching that special region, and the prospects for eventually reaching that region are very good.
To create the new isotope, the research team fired a beam of platinum ions at a carbon target. The beam current divided by the state of charge was 50 nanoamps. Since these experiments were conducted, FRIB has already scaled up the beam power to 350 nanoamps and has plans to reach up to 15,000 nanoamps.
On the other hand, new isotopes are exciting in their own right, offering the nuclear research community new opportunities to venture into uncharted territory.
“It’s not a big surprise that these isotopes exist, but now that we have them, we have colleagues who are very interested in what we can measure next,” Gade said. “I’m already starting to think about what we can do next in terms of measuring their half-life, mass, and other properties.”
Studying these quantities in previously unavailable isotopes can help inform and improve understanding of basic nuclear science.
“I still have a lot to learn,” Sherrill said. “And we’re on our way.”
For more information:
OB Tarasov et al, New isotope observations in fragmentation of Pt198 at FRIB, physical review letter (2024). DOI: 10.1103/PhysRevLett.132.072501
[ad_2]
Source link